iodine electron configuration|ion electron configuration calculator : Manila The total number of electrons in iodine is fifty-three. These electrons are arranged according to specific rules in different orbitals. . Tingnan ang higit pa r/AccountingPH Accounting Subreddit for Filipinos Students, Reviewees and CPA professionals are welcome here. For Students and Reviewees: Ask questions, post review materials, and share your experiences For CPA professionals: Share your experiences, give advices, ask for help For EVERYONE: Enjoy your stay and welcome to a material world.
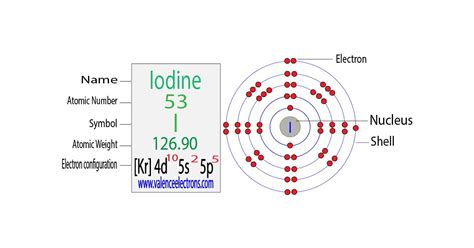
iodine electron configuration,Learn how to write the complete electron configuration of iodine (I) using two different methods: orbit and orbital. Compare the rules, formulas, and examples of each method and see the diagrams of iodine's electron arrangement. Tingnan ang higit paiodine electron configuration ion electron configuration calculatorThe total number of electrons in iodine is fifty-three. These electrons are arranged according to specific rules in different orbitals. . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular . Tingnan ang higit pa
Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of iodine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. In the iodine ground-state electron configuration, . Tingnan ang higit pa
iodine electron configurationAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital. The sub-energy levels depend on the azimuthal quantum . Tingnan ang higit pa Mar 23, 2023 Today we are going to give you all the information related to the electron configuration of the Iodine. Iodine Number of Valence Electrons. Iodine has seven Valence Electrons. What is The .
Full electron configuration of iodine: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. tellurium ← iodine → xenon. Iodine, complete electron configuration.Iodine is a nonmetal with atomic number 53 and symbol I. Its electron configuration is [Kr] 4d 10 5s 2 5p 5, with valence electrons of 7 and valency electrons of 1,3,5,7.Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), .Learn the electron configuration of iodine, a halogen with atomic number 53 and symbol I. Find out its properties, biological function, deficiency and excess effects.

Electron configuration of Iodine is [Kr] 4d10 5s2 5p5. Possible oxidation states are +1,5,7/-1. Electron Configuration. The periodic table is a tabular display of the . Learn how to write, diagram, and abbreviate the electronic configuration of iodine, a 5-period element with 53 electrons. See also the ground and excited states of .
Learn about the electron configuration of iodine, a purple-black halogen with the symbol I and atomic number 53. Find out its properties, uses, emission spectra, and .Orbital diagram. Iodine electron configuration. ← Electronic configurations of elements. I (Iodine) is an element with position number 53 in the periodic table. Located in the V period. Melting point: 113.5 ℃. Density: 4.94 g/cm 3 . Electronic configuration of the Iodine atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 .The Electron configuration of iodine is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s²4d¹⁰ 5p⁵. Iodine, also called iodine is defined as the chemical element that belongs to the periodic table. It is located in group 17, more precisely in the halogens, its atomic number is 53 and it is represented by the symbol I. .
ion electron configuration calculator Iodine is a 5-period element with a total of 53 electrons, filled in the ascending series of energy levels 1s, 2s, 2p, and so on. The s, p, d and f orbitals can hold a maximum of 2, 6, 10 and 14 electrons respectively. The configuration of I is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4d10 5s2 after filling 48 electrons entirely with respective subshells. A step-by-step description of how to write the electron configuration for Iodine (I). In order to write the I electron configuration we first need to know t.
The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Iodine is [Kr] 4d10 5s2 5p5. Possible oxidation states are +1 .
Electron Configuration and Oxidation States of Iodine. Electron configuration of Iodine is [Kr] 4d10 5s2 5p5. Possible oxidation states are +1,5,7/-1. Electron Configuration. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical .Configuration. σ g π u 4 π g 3 σ u 2. 10 . of the D state excited by the 1830 atomic line of iodine. The system further includes the diffuse emission bands in the region 2500 - 5000 with a . Schwarz, W.H.E., Inner electron excitation of iodine in the gaseous and solid phase, J. Chem. Phys., 1973, 58, 2230. Venkateswarlu, 1970 .Element Iodine (I), Group 17, Atomic Number 53, p-block, Mass 126.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.

The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ).
Electron Configuration: Electron configurations are a way of keeping track of the location of the electrons around the nucleus. The quantum number information for the electrons is grouped by energy levels, the orbitals are combined within the subshell. Answer and Explanation: 1 In this video we will write the electron configuration for I- the Iodide ion. We’ll also look at why Iodine forms a 1- ion and how the electron configuration.Write the subshell electron configuration (i.e. 1s^2 2s^2, etc.) for the Si14 atom and identify which are valence (outer shell) electrons and determine how many valence electrons there are. Write the electron configuration you would expect for iodine (Z = 53). Use a noble gas core. How do electron configurations correspond to the periodic . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .
Configurazione elettronica dello iodio. La configurazione elettronica dello iodio è 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s²4d¹⁰ 5p⁵. Lo iodio, detto anche iodio è definito come l'elemento chimico che appartiene alla tavola periodica. Si trova nel gruppo 17, più precisamente negli alogeni, il suo numero atomico è 53 ed è . Iodine is the only solid halogen at room temperature. It forms a purple gas when heated, and bears the Greek name iodine (violet). Skip to content. . Electron configuration. The electron configuration of an element describes the arrangement of electrons in the atoms of that element, and be used to predict its chemical properties .
La configuración electrónica del yodo es 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s²4d¹⁰ 5p⁵. El yodo, también llamado yodo, se define como el elemento químico que pertenece a la tabla periódica. Se encuentra en el grupo 17, más precisamente en los halógenos, su número atómico es 53 y se representa con el símbolo I. Este .
The electronic configuration of Iodine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. What is the abbreviated electronic configuration of Iodine? The abbreviated electronic configuration of Iodine is [Kr] 4d10 5s2 5p5. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas .
iodine electron configuration|ion electron configuration calculator
PH0 · unabbreviated electron configuration iodine
PH1 · ion electron configuration calculator
PH2 · iodine electron configuration shorthand
PH3 · iodine abbreviation electron configuration
PH4 · electron configuration for every element
PH5 · electron configuration chart pdf
PH6 · electron configuration chart
PH7 · electron configuration calculator
PH8 · Iba pa